Search for Cord Blood Studies
on ClinicalTrials.gov
ClinicalTrials.gov is an online registry of medical research worldwide that can be a valuable resource for patients, families, medical professionals and the general public. The searchable registry includes a “results database” that summarizes completed and terminated clinical trials.
Provided below is guidance on how to search for cord blood-related studies.
- Go to the ClinicalTrials.gov homepage at https://clinicaltrials.gov/ct2/home.
- Click the “Advanced Search” button within the “Search for Studies” box or in the menu under the “Find Studies” tab. You will be brought to a page with many options to refine your search.
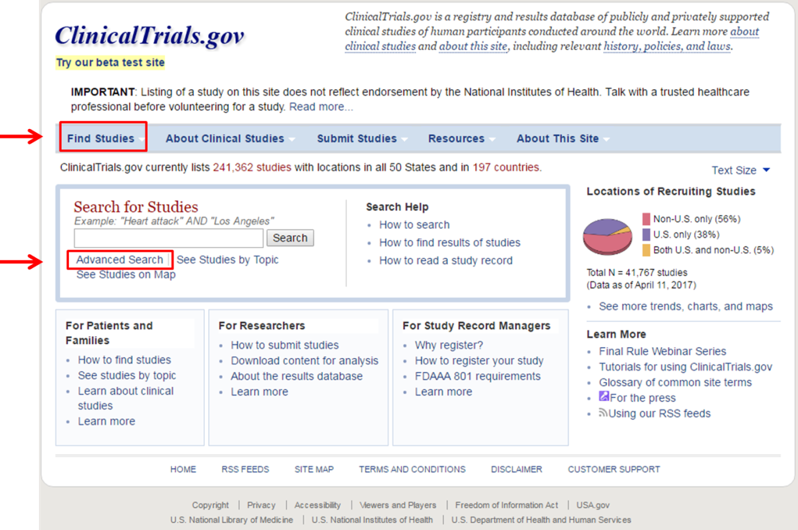
- To search, simply enter terms in which you are interested into the “Search Terms” box. You can further refine your search by criteria such as eligibility (age, gender, etc.), specific types of disease or intervention, location, phase of study, and funding source.
- For example, if you are searching for a clinical trial that is currently enrolling adults with Acute Myeloid Leukemia to undergo a Cord Blood Transplant in the state of Washington and that has funding from the NIH, a potential search could be:
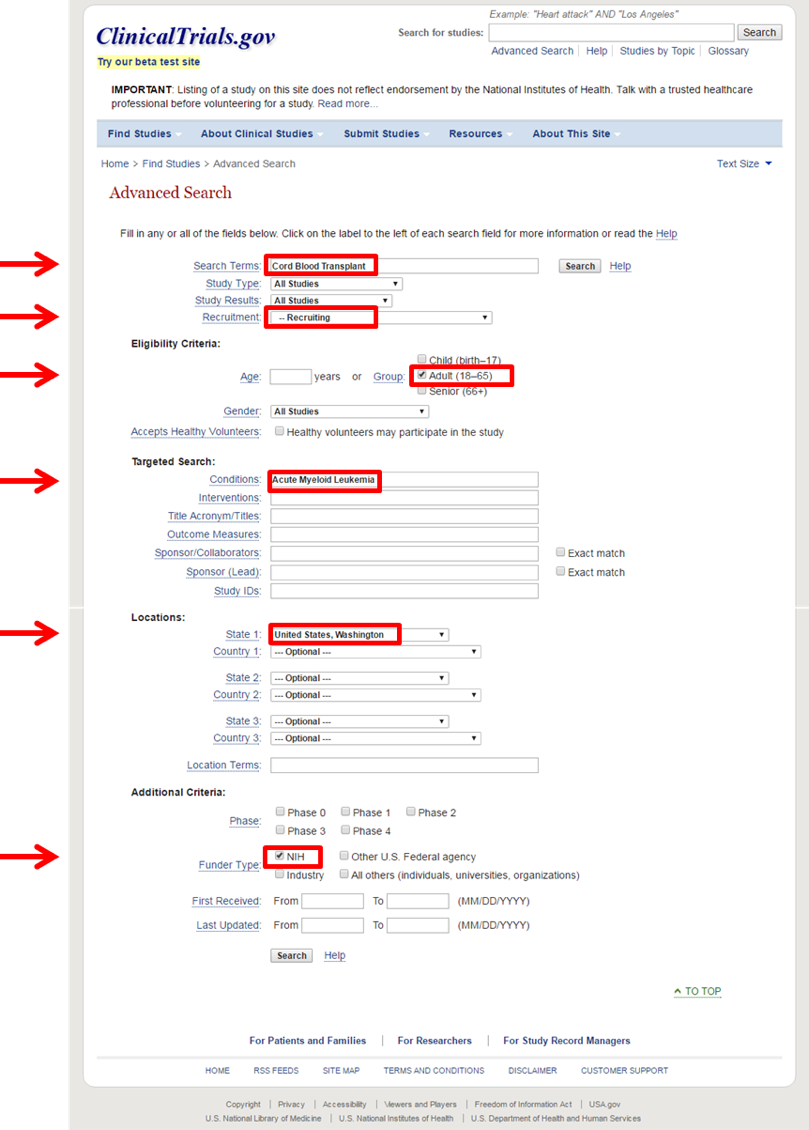
- This particular search will provide clinical trials that are open to enrollment (recruiting) for patients within the range of 18-65 years who have Acute Myeloid Leukemia who will undergo a cord blood transplant in the state of Washington, with funding from the NIH. It should be noted that a listing of a clinical trial on this site does not reflect endorsement from the National Institutes of Health and does not necessarily indicate eligibility. Final eligibility is decided by the study sponsors and investigators.
- The example search, above, provides four studies that meet the criteria:

- For more information on these studies, simply click on the study title.
Additional Information about ClinicalTrials.gov
The ClinicalTrials.gov website was initially created as part of the Food and Drug Administration Modernization Act of 1997 (FDAMA) to create a registry of federally and privately funded clinical trials conducted with investigational new drug applications. Requirements to register clinical trials with ClinicalTrials.gov were expanded after Congress passed the FDA Amendments Act of 2007 (FDAAA).
For further information on the background of ClinicalTrials.gov, please visit https://clinicaltrials.gov/ct2/about-site.
|