Research Advances
By Karen Ballen, MD, Michael Horwitz, MD, Junya Kanda, MD, PhD, and Elisabetta Xue, MD
Updated: February, 2024
RECENTLY PUBLISHED ARTICLES
post transplant: Non: UCB
A Phase I Dose-Escalation Clinical Trial to Assess the Safety and Efficacy of Umbilical Cord-Derived Mesenchymal Stromal Cells in Knee Osteoarthritis
Jose Matas et al, Stem Cells Transl Med, Feb 2024
Background: Osteoarthritis is among the most common degenerative joint diseases. In a preclinical setting, the intra-articular injection of Cellistem, an umbilical-derived mesenchymal stromal cells product, exerted a dose-dependent cartilage protective effect and was associated with decreased inflammatory and degenerative response and decreased Th1 and Th17 lymphocytes infiltration.
Methods: The authors performed a dose-escalation phase I clinical trial evaluating the safety of Cellistem. Three sequential cohorts were enrolled: low-dose group (2 × 106 cells), medium-dose group (20 × 106), and high-dose group (80 × 106). Mesenchymal stromal cells for Cellistem were isolated and characterized based on their immunophenotype profile, tri-differentiation potential, and immunosuppressive capacities, thrombospondin 2 production, and karyotype analysis, according to the ISCT criteria. Intra articular injection contained MSCs diluted in saline with 5% AB+ plasma.
Results: Forty patients were enrolled, 16 in each of the low and the medium dose groups and 8 in the high dose group. All patients in the high-dose group experienced injection-related swelling in the knee joint. Most common adverse event was knee pain after injection, with more intense and prolonged symptoms in the high dose cohort, therefore the recruitment for this dose level was discontinued. No serious adverse events, septic arthritis, disability, or hospitalization were observed. No treatment-related abnormal structural changes were reported in MRI analysis at 6 months of follow-up. According to Western Ontario and Mc Master Universities Arthritis Index (WOMAC), patients from all dose level groups reported significant improvements in pain and function compared with baseline after 3 and 6 months; interestingly, the improvements were higher in patients treated with both medium and low dose as compared to high dose.
The authors concluded that the intra-articular injection of low-to-medium dose of Cellistem was safe in patients with mild and symptomatic knee osteoarthritis and determined a significant clinical improvement in terms of pain, function and quality of life.
Trial registration number: NCT03810521.
Hospitalization and Healthcare Resource Utilization of Omidubicel-Onlv versus Umbilical Cord Blood Transplantation for Hematologic Malignancies: Secondary Analysis from a Pivotal Phase 3 Clinical Trial
Navneet S Majhail et al, Transplant Cell Ther. https://doi.org/10.1016/j.jtct.2023.09.004
Background: A phase 3 trial (NCT02730299) of omidubicel-onlv, a nicotinamide-modified allogeneic hematopoietic progenitor cell therapy manufactured from a single umbilical cord blood (UCB) unit, showed faster hematopoietic recovery and reduced rate of infections compared with patients randomized to standard UCB.
Methods: The authors conducted a prospective secondary analysis of the trial to characterize resource utilization in the first 100 days post-transplantation with omidubicel-onlv (study group, n=52) compared with UCB (control group, n=56). The analysis examined hospital length of stay, hospital care setting, visits by provider type, rate of transfusions, and readmissions among both cohorts from day 0 to day 100 post-transplantation. The two groups did not differ for baseline characteristics except for higher proportion of females (52% versus 37%) and older median age (40 years vs 36 years) in the study group.
Results: In the first 100 days post-transplantation, patients in study group had a shorter average total hospital length of stay (average 41.2 days vs 50.8 days; p = .027) and had more days alive and out of the hospital (mean, 55.8 days versus 43.7 days; p = .023). During primary hospitalization (time from transplantation to discharge), fewer patients in the study group required intensive care unit admission (9.6% vs 23.2%) and spent fewer days in the ICU (average 0.4 day versus 4.7 days; p = .028) and transplant unit (average 25.3 days vs 32.9 days; P = .022) compared with those receiving UCB. Patients receiving omidubicel-onlv required fewer outpatient consultant visits (6.8 vs 20.1, p=0.15). Also, study group required fewer transfusions of platelet (18.3 vs 23.7, p=0.51) and red blood cells transfusion (6.4 vs 11.2, p=0.003).
The authors concluded that the faster hematopoietic recovery in omidubicel-onlv patients was associated with significantly shorter hospital stay and reduced healthcare resource use compared with standard UCB.
Safety of romiplostim administered immediately after cord-blood transplantation: a phase 1 trial
Naoki Kurita et al, Ann Hematol DOI: 10.1007/s00277-023-05410-3
Background: Delayed hematopoietic recovery and high transfusion requirements are the major limitations of cord-blood transplant. Romiplostim, a thrombopoietin-receptor agonist, promotes multilineage hematopoiesis.
Methods: This phase 1 dose-escalation study was designed to evaluate the safety and feasibility of romiplostim administration early after single unit cord blood transplant. Six adult patients undergoing either myeloablative or reduced intensity transplant for hematologic malignancies were enrolled. Graft versus host prophylaxis included calcineurin inhibitor with mycophenolate mofetil. Romiplostim was administered subcutaneously within 7 days from transplant, at an initial dose of 5 µg/kg or 10 µg/kg, then once a week for 14 weeks or until platelet recovery.
Results: A median of 6 romiplostim administrations were given at the maximum dose of 20 µg/kg. Patients were followed for a median of 34 months. One patient had to discontinue due to the development of an infectious adverse event, not related to romiplostim. Romiplostim-related adverse events included bone pain (3/6) and injection site reaction (1/6). Non-hematological grade ≥ 3 toxicities were observed in four patients: febrile neutropenia was the most common (4/6). All patients achieved neutrophil engraftment after a median of 14 days (range, 12-32). Patients received a median of 10.5 platelet transfusion (range, 4–30). Platelet counts ≥ 50 × 109 /L were recorded in all patients except for one who died on day 48; the median time for platelet ≥ 50 × 109 /L was 34 days (range, 29-98). No disease relapse, thrombo-embolic events, or bone marrow fibrosis were observed.
The authors concluded romiplostim administration early after CBT was safe, but larger, phase 2 trials will be required for efficacy evaluation.
Trial registration number: UMIN000033799.
Randomized, double-blinded, placebo-controlled trial of allogeneic cord blood T-regulatory cells for treatment of COVID-19 ARDS
Douglas E Gladstone et al, Blood Adv DOI: 10.1182/bloodadvances.2022009619
Background: T reg cells can suppress excessive immune responses as observed in COVID-19 associated acute respiratory distress syndrome (ARDS)
Methods: A phase 1, randomized, multicenter, double-blinded, placebo-controlled trial was conducted in patients with COVID-19 ARDS to examine the safety and early efficacy of CK0802, an off-the-shelf, cryopreserved, allogeneic, umbilical cord derived Treg cell product. No HLA matching was required, and participants were allowed to received cellular products from multiple donors. Eligibility criteria included diagnosis of SARS-CoV-2 infection, moderate-to-severe ARDS, intubated for less than 120 hours, age ≥18 years, and ability to obtain informed consent. The CK0802 product was generated from 8 manufacturing campaigns, with an average of 4429 × 106 Treg cells per run, and subsequently cryopreserved. All products met the release criteria of CD4+CD25+>60% and CD4-CD8+<10%. The participants were randomized in a 1:1:1 ratio to placebo, 100 million Tregs (CK0802-100), or 300 million Tregs (CK0802-300) per infusion on day 0, day 3, and day 7. Two primary outcomes were grade ≥3 regimen-related NCI-CTCAE V4.0 toxicity within 48 hours of infusion, and S28= patient alive and not intubated 28 days after the first infusion.
Results: A total of 45 participants were enrolled, 15 per cohort, most of whom were on vasopressor, and previously received remdesivir and/or steroid. The median follow-up period from the first infusion to the last follow-up was 45-days (IQR, 23-235). Thirteen patients died: 4 in the placebo group, 2 in the CK0802-100 group and 2 in the CK0802-300 group. None had a grade ≥3 toxicity related to the investigational agent. S28 was achieved in 9, 9, and 6 patients in the placebo, CK0802-100, and CK0803-300 arms, respectively. Bayesian regression analyses demonstrated 89.7% probability of beneficial effect on S28 for CK0802-100 vs placebo (95% CI, −0.71 to 3.54), and 28.4% for CK0802-300 vs placebo (95% CI, −2.7 to 1.5).
The authors concluded that infusion of multiple doses of cryopreserved, allogeneic, and non–HLA-matched regulatory T cells were overall safe in critically ill patients with COVID-19 associated ARDS; larger studies will be needed to evaluate efficacy of this regimen. Also, the authors postulated that, with a multifaceted mechanism of action including secretion of the anti-inflammatory cytokine and acting as a cytokine sink for the proinflammatory cytokines, the balance-restorative intervention of T regs should not be affected by specific SARS-CoV-2 variants, and therefore could find wider application for treatment of virus-induced ARDS.
Trial registration number: NCT04468971.
Safety, immunological effects and clinical response in a phase I trial of umbilical cord mesenchymal stromal cells in patients with treatment refractory SLE
Diane L Kamen et al Lupus Sci Med DOI: 10.1136/lupus-2022-000704
Abstract
Background: This study aimed to evaluate the immunosuppressive properties of umbilical cord derived MSCs in patients with systemic lupus erythematosus refractory to 6 months of immunosuppressive therapy.
Methods: The MSCs were derived from umbilical cords of two healthy donors under FDA IND 16377. Potential donors were excluded if they had personal or family history of autoimmune disease or any positives on infectious and autoimmune testing.
Six women with a SLE disease activity index (SLEDAI) >6, having failed standard of care therapy, received one single intravenous infusion of 1×106 MSCs/kg. Current immunosuppressants were not discontinued, but the treating physicians were allowed to adjust dose of corticosteroid for symptom management. The SLE Responder Index (SRI) 4 assessed at 24 weeks was the primary endpoint: a decrease in the SLEDAI of at least 4, no new BILAG As or two BILAG Bs and no increase in the Physicians Global Assessment >0.3 were required to be considered responsive; inability to taper prednisone to 10 mg or less by 20 weeks was considered a treatment failure.
Results: The six participants had variable lupus manifestations at time of enrolment. Of six patients, five (83.3%; 95% CI 35.9% to 99.6%) achieved the clinical endpoint of an SRI of 4. No serious adverse events attributed to MSC were observed, and all adverse events were grade 2 or less. Despite no major changes in the T cell compartment other than increase of Helios+ T regs in two patients, the authors observed a significant change in B cell population, with reductions in CD27IgD double negative B cells, switched memory B cells and activated naïve B cells, with increased transitional B cells in the five responders. GARP-TGFβ complexes were significantly increased. The B cell changes and the GARP-TGFβ increases significantly correlated with changes in SLEDAI scores. Of note, one out of five responders remained with minimal disease activity for four years after the infusion, whereas other four experienced a disease flare 18 to 30 months after infusion. Currently, a phase II double-blind multicenter study evaluating efficacy of UB-MSC in SLE patients is in progress.
Trial registration number: NCT03171194.
Improved Outcomes of UM171-Expanded Cord Blood Transplantation Compared with Other Graft Sources: Real-World Evidence
Cohen S, et al. Blood Advances, 2023
Hôpital Maisonneuve Rosemont, Université de Montréal, Montreal, Quebec, Canada.
A phase I-II trial of UM171-expanded CB transplants demonstrated safety and favorable preliminary efficacy. The aim of the current analysis was to retrospectively compare results of the phase I-II trial to those after unmanipulated CB and matched unrelated donor (MUD) transplants.
Methods: Data from recipients of CB and MUD transplants were obtained from the CIBMTR database. Patients were directly matched for the number of prior allogeneic hematopoietic stem cell transplants (alloHCT), disease and refined Disease Risk Index. Patients were further matched by propensity score for age, comorbidity index and performance status.
Findings: Overall, 137 CIBMTR (67 CB, 70 MUD) and 22 UM171-expanded CB patients were included. NRM at 1 and 2 years was lower, PFS and GRFS at 2 years and OS at 1 year were improved for UM171-expanded CBs compared to CB controls. Compared to MUD controls, UM171 patients had lower 1- and 2-year NRM, higher 2-year PFS, and higher 1- and 2-year GRFS. Furthermore, UM171-expanded CB recipients experienced less grades III-IV acute GVHD and chronic GVHD compared to MUD graft recipients.
Conclusions: Compared to real-world evidence with CB and MUD alloHCT, this study suggests that UM171-expanded CB recipients may benefit from lower NRM and higher GRFS.
Intrabone Transplantation of a Single Unwashed Umbilical Cord Blood Unit with Antithymocyte Globulin-Free and Sirolimus-Based Graft-versus-Host Disease Prophylaxis: Fast Immune Reconstitution and Long-Term Disease Control Patients with High-Risk Diseases.
Giglio F, et al. Transplantation and Cellular Therapy, 2023
IRCCS San Raffaele Hospital, Milan, Italy.
The aim of this retrospective analysis was to examine the safety and efficacy of intrabone transplantation of a single unwashed cord blood unit in an antithymocyte globulin-free, sirolimus-based graft-versus-host disease prophylaxis platform.
Methods: Authors collected data for all consecutive UCBTs infused intrabone (IB) and unwashed at San Raffaele Hospital in Milan between 2012 and 2021.
Findings: Thirty-one consecutive UCBTs were identified. At the time of cryopreservation, the median CD34+ cell count was 1 × 105/kg (range, 0.6 to 12.0 × 105/kg) and the median total nucleated cell (TNC) count was 2.8 × 107/kg (range, 1.48 to 5.6 × 107/kg). No adverse events were related to the IB infusion at bedside under short-conscious periprocedural sedation or to the no wash technique. After thawing, median CD34+ cell and TNC counts were 0.8 × 105/kg (range, 0.1 to 2.3 × 105/kg) and 1.42 × 107/kg (range, 0.69 to 3.2 × 107/kg). The median time to engraftment was 27 days for neutrophils and 53 days for platelets. One patient experienced graft rejection and was subsequently rescued with a salvage transplantation. The median time to a CD3+ cell count >100/μL was 30 days. The 2-year cumulative incidence of moderate-to-severe chronic GVHD (cGVHD) was 11.8%. At 2 years, overall survival was 52.7% (95% CI, 33% to 69%), relapse incidence was 30.7% (95% CI, 13.7% to 49.6%), and transplantation-related mortality was 29% (95% CI, 14.3% to 45.6%).
Conclusions: IB infusion of a single cord blood unit was feasible, with no adverse reactions related to the no wash/IB infusion, low rates of cGVHD and disease relapse, and rapid immune reconstitution.
Outcomes of graft failure after umbilical cord blood transplantation in acute leukemia: a study from Eurocord and the Acute Leukemia Working Party of the EBMT.
Baron F, et al. Bone Marrow Transplantation, 2023
University and CHU of Liege, GIGA-I3, Liege, Belgium
The outcomes of patients who experienced graft failure after CBT were analyzed.
Methods: Inclusion criteria were patients (age ≥ 18 years) experiencing graft failure after unrelated CBT (single or double) between 2005 and 2016, for acute myelogenous leukemia (AML) or acute lymphoblastic leukemia (ALL), no prior allogeneic or autologous transplantation, no other stem cell product.
Findings: The study included 87 patients. At 1-year, cumulative incidence of relapse and non-relapse mortality (NRM) was 35% and 37%, respectively. One-year overall survival (OS) and progression-free survival (PFS) was 40% and 29%, respectively. Forty-six patients underwent a salvage second transplantation with 1-year and 2-year OS and PFS from second transplantation 41% and 34% for OS, and 37% and 34% for PFS, respectively. In multivariate analysis, complete remission (CR) at CBT and reduced-intensity conditioning were associated with better OS.
Conclusions: In this retrospective study, approximately one-quarter of patients experiencing graft failure after CBT remained alive without relapse 2 years later.
Dynamic comparison of early immune reactions and immune cell reconstitution after umbilical cord blood transplantation and peripheral blood stem cell transplantation.
Zhao X, et al. Frontiers in Immunology, 2023
University of Science and Technology of China, Hefei, Anhui, China
The differences in the immune cell reconstitution and the immune reactions during initial stages post-transplantation are not well established between UCBT and PBSCT.
Methods: Authors analyzed the differences in the immune reactions during the early stages such as pre-engraftment syndrome (PES), engraftment syndrome (ES), and acute graft-versus-host disease (aGVHD) and the immune cell reconstitution between the UCBT and the PBSCT group of patients. They enrolled a cohort of patients that underwent UCBT or PBSCT and healthy controls (n=25 each).
Findings: The incidences of early immune reactions such as PES, ES, and aGVHD were significantly higher in the UCBT group compared to the PBSCT group. Furthermore, in comparison with the PBSCT group, the UCBT group showed higher proportion and numbers of naïve CD4+ T cells, lower proportion and numbers of Tregs, higher proportion of CD8+ T cells with increased activity, and higher proportion of mature CD56dim CD16+ NK cells during the early stages post-transplantation. Moreover, the plasma levels of GM-CSF were significantly higher in the UCBT group compared to the PBSCT group in the third week after transplantation. Overall, our findings demonstrated significant differences in the post-transplantation immune cell reconstitution between the UCBT and the PBSCT group of patients.
Conclusions: These characteristics were associated with significant differences between the UCBT and the PBSCT groups regarding the incidences of immune reactions during the early stages post transplantation.
Epitope Mismatch at HLA-DRB1 Associates with Reduced Relapse Risk in Cord Blood Transplantation for Standard-Risk Hematologic Malignancy.
Morita-Fujita M, et al. Transplantation and Cellular Therapy, 2023
Kyoto University, Kyoto, Japan
Given that HLA molecules contain epitopes comprising polymorphic amino acids that determine their immunogenicity, authors investigated associations between epitope-level HLA mismatches and relapse following single-unit CBT.
Methods: A total of 492 patients with hematologic malignancies who underwent single-unit, T cell-replete CBT were included in this multicenter retrospective study. HLA epitope mismatches (EMs) were quantified using HLA matchmaker software from donor and recipient HLA-A, -B, -C, and -DRB1 allele data.
Findings: Patients were dichotomized by median EM value and divided into 2 groups: patients who underwent transplantation in complete/partial remission and others. The median number of EMs in the graft-versus-host direction (GVH-EM) was 3 (range, 0 to 16) at HLA class I and 1 (range, 0 to 7) at HLA-DRB1. Higher HLA class I GVH-EM was associated with increased nonrelapse mortality (NRM) in the advanced stage group, with no significant advantage for relapse in either stage. In contrast, higher HLA-DRB1 GVH-EM was associated with better disease-free survival in the standard stage group, which was attributed to lower relapse risk.
Conclusions: High HLA-DRB1 GVH-EM may lead to potent GVT effects and a favorable prognosis following CBT, especially in patients who underwent transplantation at the standard stage. This approach may facilitate appropriate unit selection and improve the overall prognosis of patients with hematologic malignancies who undergo CBT.
Effect of Graft-versus-Host Disease on Post-Transplantation Outcomes following Single Cord Blood Transplantation Compared with Haploidentical Transplantation with Post-Transplantation Cyclophosphamide for Adult Acute Myeloid Leukemia.
Konuma T, et al. Transplantation and Cellular Therapy, 2023
The Institute of Medical Science, The University of Tokyo, Tokyo, Japan
The objective of this retrospective study was to compare the effect of acute GVHD and chronic GVHD on post-transplantation outcomes between recipients of CBT and recipients of PTCy-haplo-HCT.
Methods: Authors retrospectively evaluated the effect of acute and chronic GVHD on post-transplantation outcomes following CBT and PTCy-haplo-HCT in adults with AML (n = 1981) between 2014 and 2020 using a Japanese registry database.
Findings: In univariate analysis, the probability of overall survival was significantly greater in patients who developed grade I-II acute GVHD and limited chronic GVHD among CBT recipients, but these effects were not significant among PTCy-haplo-HCT recipients. In multivariate analysis, in which the development of GVHD was treated as a time-dependent covariate, the effect of grade I-II acute GVHD on reducing overall mortality differed significantly between CBT and PTCy-haplo-HCT (adjusted hazard ratio [HR] for CBT, 0.73, 95% confidence interval [CI], 0.60 to 0.87; adjusted HR for PTCy-haplo-HCT, 1.07; 95% CI, 0.70 to 1.64; P for interaction = 0.038).
Conclusions: Grade I-II acute GVHD was associated with a significant improvement in overall mortality in adults with AML receiving CBT but not in recipients of PTCy-haplo-HCT.
Impact of allele-level HLA matching on outcomes after double cord blood transplantation in adults with malignancies.
Fatobene G, et al. Blood Advances, 2023
Universidade de Sao Paulo, Sao Paulo, Brazil
In this study, authors report the impact of allele-level HLA matching on the outcomes of a large double UCBT (dUCBT) cohort.
Methods: Authors included 963 adults with hematologic malignancies, with available allele-level HLA matching at HLA-A, -B, -C, and -DRB1, receiving dUCBT between 2006 to 2019. Assignment of donor-recipient HLA match was performed considering the unit with the highest disparity with the recipient. Three hundred ninety-two patients received dUCBT with 0 to 3 MM and 571 with ≥4 allele MM.
Findings: For recipients of dUCBT with 0 to 3 MM, day-100 and 4-year TRM were 10% and 23%, respectively, compared with 16% and 36% for those with ≥4 MM. A higher degree of allele MM was also associated with the worse neutrophil recovery and lower incidence of relapse; no significant effect on graft-versus-host disease was observed. Patients receiving units with 0 to 3 MM had a 4-year OS of 54% compared with 43% for those receiving units with ≥4 MM. The inferior OS associated with higher HLA disparity was only partially mitigated by increased total nucleated cell doses.
Conclusions: The results confirm that allele-level HLA typing is a significant factor for OS after dUCBT, and units with ≥4 MM (≤4/8 HLA-matched) should be avoided if possible.
A Portrait of Cord Blood Units Distributed for Transplantation from Canadian Blood Services' Cord Blood Bank: First Analysis.
Parmar G, et al. Current Oncology, 2022
Canadian Blood Services, Ottawa, Canada
The Canadian Blood Services Cord Blood Bank (CBS CBB) was created to improve access to stem cell products for transplantation for patients across ethnic groups. An analysis of distributed units is needed to assess the effectiveness of the bank to meet the needs of patients from different ethnic groups.
Methods: A descriptive analysis was performed on all cord blood units distributed from the CBS' CBB as of 30 June 2022.
Findings: Distribution of the first 60 units based on CBS' CBB inventory has been linear over time. A similar proportion of cord blood unit (CBU) recipients were pediatric or adult. More than half of the cord blood units (56.7%) were distributed to recipients outside of Canada, and CBUs were used to treat a broad range of hematologic and immune disorders. 43.3% of distributed CBUs were of non-Caucasian ethnicity and 18% were from donors self-reporting as multi-ethnic. The mean total nucleated cell counts and total CD34+ cell counts were 1.9 ± 0.1 × 109 cells and 5.3 ± 0.5 × 106 CD34+ cells, respectively. CD34+ cells per kg (recipient weight) varied significantly between pediatric (age 0-4), adolescent (age 5-17) and adult recipients (age 18 and older) (3.1 ± 0.5, 1.4 ± 0.5 and 0.9 ± 0.07 × 105 CD34+ cells/kg, respectively). HLA matching was 6/6 (15%), 5/6 (47%) or 4/6 (38%).
Conclusions: The CBS' CBB has facilitated the utilization of banked units for patients across a broad range of ages, geographic distribution, ethnicity, and diseases. Distributed units were well matched for HLA alleles and contained robust cell counts, reflecting a high-quality inventory with significant utility.
Clinical Outcomes of Umbilical Cord Blood Transplantation Using Ex Vivo Expansion: A Systematic Review and Meta-Analysis of Controlled Studies.
Saiyin T, et al. Transplantation and Cellular Therapy, 2023
University of Ottawa, Ottawa, Ontario, Canada
Ex vivo culture strategies have been increasingly evaluated in controlled studies, but their impact on transplantation-related outcomes remains uncertain owing to the small patient numbers in these studies, necessitating an updated systematic review and meta-analysis.
Methods: A systematic literature search was conducted using the MEDLINE, Embase, and Cochrane databases to March 18, 2022. Nine cohort-controlled phase I to III trials were identified, and data of 1146 patients undergoing umbilical cord blood transplantation (UCBT) were analyzed (308 ex vivo expanded and 838 unmanipulated controls). Expansion strategies involved cytokine cocktails plus the addition of small molecules (UM171, nicotinamide [NiCord], copper chelation, Notch ligand, or Stem regenin-1 [SR-1]) and coculture with mesenchymal stromal cells in a single-unit transplant strategy (5 studies) or a double-unit transplant strategy with 1 unmanipulated unit (4 studies).
Findings: The included trials reported a median ex vivo expansion of CD34+ cells from 28-fold to 330-fold. Eight of the 9 studies demonstrated a significantly faster time to initial neutrophil and platelet engraftment using expanded cells compared with controls. Studies using UM171 and NiCord in single-unit UCBT and SR-1 or NiCord double-unit UCBT demonstrated long-term donor chimerism of the expanded unit at 100 days to 36 months post-transplantation in all single-unit recipients and in 35% to 78% of double-unit recipients. Our meta-analysis revealed a lower risk of death at the study endpoint in patients who received ex vivo expanded grafts (odds ratio [OR], 0.66; 95% confidence interval [CI], 0.47 to 0.95; P = 0.02), while the risk of grade II-IV acute graft-versus-host disease was unchanged (OR, 0.79; 95% CI, 0.58 to 1.08; P = 0.14).
Conclusions: This review indicates that UCBT following ex vivo expansion can accelerate initial engraftment. Durable donor chimerism can be achieved after transplanting cord blood units expanded using NiCord, UM171, or SR-1; however, long term outcomes remain unclear.
Cord Blood Transplantation for Nonmalignant Disorders: Early Functional Immunity and High Survival
Martinez C et al, Blood Advances, 2022
Baylor College of Medicine, Texas Children's Hospital, Houston, Texas, USA
There is no consensus about the best donor for children with non-malignant disorders and immune deficiencies in the absence of a matched related donor (MRD).
Methods: Authors evaluate the 2-year overall survival after unrelated cord blood transplant (UCBT) in patients with non-malignant disorders from 2009-2020 enrolled on a prospective clinical trial using either 5/6 or 6/6 umbilical cord blood as cell source. Patients receive a fully ablative busulfan, cyclophosphamide and fludarabine without serotherapy. 55 children were enrolled, median age 5 months (range, 1-111 months).
Findings: The overall survival (OS) at 2 years was 91% (95% CI:79-96%) with a median follow up of 4.3 years. The median time to neutrophil and platelet recovery were 17 days (range 5-39 days) and 37 days (range 20-92 days), respectively. All but one evaluable patient achieved full donor chimerism. The cumulative incidence of aGvHD grade II-IV by day 100 was 16% (n=9). All patients with viral infections at the time of transplant cleared the infection at a median time of 54 days (range 44-91 days). All evaluable patients have had correction of their immune or metabolic defect.
Conclusions: In the absence of a MRD, UCBT following myeloablative conditioning without serotherapy is an excellent curative option in young children with non-malignant disorders.
Outcomes of Subsequent Neoplasms after Umbilical Cord Blood Transplantation in Europe
Rafii H et al, Blood Advances, 2022
Monacord, Centre Scientifique de Monaco, Monaco.
Subsequent neoplasms (SNs) compromise long-term survivors after hematopoietic cell transplantation.
Methods: Authors performed a retrospective analysis of SNs in a cohort of 10,358 recipients of unrelated cord blood transplants (UCBT) reported to Eurocord/EBMT registries from 1988 to 2018.
Findings: A total of 233 patients developed SNs. Median age at UCBT was 31 years (y) (0.3-69), and 84 were pediatric patients. Three groups of SNs were observed. Post-transplant lymphoproliferative disorders (PTLD) were reported in 145 patients in a median of 4 months after UCBT. Of these, 9/145 patients died from relapse, 83/145 from PTLD, and 24/145 from transplant-related causes. At last follow-up, 29/145 were alive; 5y-overall survival (OS) after PTLD diagnosis was 21±3%. Acute leukemia/myelodysplasia (AL/MDS) was diagnosed in 23 patients in a median of 28 months after UCBT and included 3 donor-cell AL. Four of 23 patients died from relapse of primary disease, 8/23 from progression of SNs, and 4/23 from TRM. Seven patients were alive at last follow-up; 5y-OS after AL/MDS diagnosis was 36±10%. Solid tumors (ST) were reported in 65 patients in a median of 54 months after UCBT. Most common tumor sites were lung, thyroid, bone and soft tissue. A total of 33/65 patients died (26 due to ST, 6 to relapse of primary disease, 1 cause missing). At last follow-up, 32/65 patients were alive; 5y-OS after the diagnosis of ST was 51±6%.
Conclusions: Despite their poor outcomes, SNs that occur after UCBT are extremely rare. Identification of associated risk factors and early detection may help to improve OS.
Comparable Survival Outcomes with Haploidentical Stem Cell Transplantation and Cord Blood Transplantation
Sugita J et al. Bone Marrow Transplantation, 2022
Hokkaido University Hospital, Sapporo, Japan
HLA-haploidentical stem cell transplantation using post-transplant cyclophosphamide (PTCy-haplo) and unrelated cord blood transplant are alternative to HLA-matched stem cell transplantation.
Methods: Authors conducted a matched-pair analysis of PTCy-haplo and UCBT using the Japanese registry data. They identified 136 patients aged between 16 and 69 years who received PTCy-haplo as their first transplantation for acute leukemia or myelodysplastic syndromes. Control group included 408 UCBT recipients selected to match the PTCy-haplo group.
Findings: Overall and relapse-free survival probabilities at 2 years were comparable between the PTCy-haplo and UCBT groups: 55% vs. 53% for overall survival (p = 0.46), and 47% vs. 48% for relapse-free survival (p = 0.79), respectively. The cumulative incidence of relapse was significantly higher (43% vs. 29%, respectively, p = 0.006), while the cumulative incidence of non-relapse mortality (NRM) was significantly lower (9% vs. 23%, respectively, p < 0.001) in the PTCy-haplo group. The cumulative incidence of grade II-IV acute GvHD was lower in the PTCy-haplo group compared to the UCBT group, while those of grade III-IV acute GvHD and chronic GvHD were not statistically different between the two groups.
Conclusions: Both PTCy-haplo and UCBT are viable alternatives to HLA-matched stem cell transplantation.
Single Cord Blood Transplantation Versus HLA-Haploidentical-related Donor Transplantation Using Posttransplant Cyclophosphamide in Patients With Hematological Malignancies
Wada F et al, Transplantation, 2022
Kyoto University, Kyoto, Japan
Unrelated cord blood (UCB) and haploidentical related donor transplantation using post-transplant cyclophosphamide (PTCy-haplo) have become alternative options to treat patients with hematological malignancies without an HLA-matched donor.
Methods: Authors conducted a retrospective study using registry data from the Kyoto Stem Cell Transplantation Group for patients who received allogeneic transplantation using a single UCB unit (n = 460) or PTCy-haplo (N = 57) between 2013 and 2019.
Findings: Overall survival in the UCB group was comparable to that in the PTCy-haplo group (hazard ratio, 1.00; 95% confidence interval, 0.66-1.52), although neutrophil and platelet engraftment were significantly delayed. Non-relapse mortality risk and the incidence of graft-versus-host disease in the UCB group were also comparable to those in the PTCy-haplo group. Although the relapse risk was similar between the UCB group and the PTCy-haplo group regardless of the disease risk, acute myeloid leukemia patients benefit from UCB transplant with a significantly lower relapse rate (hazard ratio, 0.38; 95% confidence interval, 0.18-0.76).
Conclusions: UCB transplant gives outcomes comparable to PTCy-haplo transplant, and both UCB and PTCy-haplo units are suitable as alternative donor sources.
The Safety and Efficacy of Umbilical Cord Blood Mononuclear Cells in Individuals with Spastic Cerebral Palsy: a Randomized Double-Blind Sham-Controlled Clinical Trial
Zarrabi M et al, BMC Neurology, 2022
Royan Institute for Stem Cell Biology and Technology, Tehran, Iran
In this multi-center, randomized, double blind, placebo-controlled study, the authors assessed the safety and efficacy cord blood-derived mononuclear cell in pediatric patients with cerebral palsy.
Methods: Patients with cerebral palsy between 4 and 14 years of age were included. Patients in the study arm were tested for HLA and received a single 5 × 106 /kg dose of HLA-matched CB-mononuclear cells intrathecally. Primary endpoint was change in gross motor function measure (GMFM)-66 from baseline to one year after treatment. Adverse events were safety endpoint. Other scores were also evaluated, including modified Ashworth scale (MAS), pediatric evaluation of disability inventory (PEDI), and quality of life.
Results: Seventy-two patients were treated, 36 in each group. Other than headaches right after intrathecal administration, no side effects were observed during follow-up. Authors observed more prominent improvement in GMFM-66 score in the study group (+9.62) than in the control arm (β:7.10); mean MAS was reduced in individuals in the study group (-0.87) and control group (β: -0.58). Mean PEDI and quality of life scores were higher in the study group than in the placebo group. Also, imaging data suggested improvements in white matter structure only in the study group.
Conclusions: This trial showed that intrathecal injection of cord blood-derived mononuclear cells was safe and potentially effective in children with cerebral palsy.
Systemic Administration of Allogeneic Cord Blood Mononuclear Cells in Adults with Severe Acute Contusion Spinal Cord Injury: Phase 1/2a Pilot Clinical Study-Safety and Primary Efficacy Evaluation
Smirnov V et al, World Neurosurgery, 2022
Sklifosovsky Research Institute of Emergency Care, Moscow, Russian Federation
In this Phase I trial, the authors evaluated the safety and efficacy of systemic administration of cord blood mononuclear cells in patients with acute severe spinal cord contusion.
Methods: Safety was the primary endpoint, whereas restoration of motor and sensory function in lower limbs within a 1-year period was secondary. Ten patients with acute contusion spinal cord injury were enrolled between 2015 and 2017 and received a total of four weekly infusions of AB0 group and rhesus-matched, non-HLA matched, cord blood-derived mononuclear cells. Infusion was given after primary surgery, starting within 3 days after injury, at the dose of 1.48 × 107/kg. All patients were followed up for 12 months after infusion.
Results: Despite an overall 419 adverse events, only 2 were estimated as possibly related to the cellular therapy administration, which were transitory hyperthermia, both categorized as mild, whereas the remaining 417 were related to spinal cord injury or other concomitant diseases. No patients developed GVHD or immunization against the cellular product. Neurological deficit level improvement was seen in all but one patient, whereas significant functional restoration of lower limb motor function was seen in six cases. Two patients showed complete restoration of spinal cord function.
Conclusions: The authors concluded that intravenous administration of mononuclear cells from umbilical cord blood is safe and might potentially improve neurological deficit in patients with contusion spinal cord injury.
Human Umbilical Cord Mesenchymal Stem Cells for Psoriasis: a Phase 1/2a, Single-Arm Study
Cheng L et al, Signal Transduction Targeted Therapy, 2022
Institute of Reproductive and Stem Cell Engineering, Changsha, China
The authors investigated the immunomodulatory and clinical effect of cord blood-derived mesenchymal stem cells (MSCs) in patients with psoriasis, a chronic immune-mediated systemic disease that has no definitive treatment.
Methods: In this Phase 1/2a clinical trial, 17 patients with psoriasis were enrolled and received cord blood-derived MSC systemic infusions. Adverse events and laboratory parameters were evaluated. Scores, including psoriasis area severity index (PASI), and physician global assessment (PGA) were analyzed for efficacy evaluation.
Results: Patients were followed for 6 months after infusion. No major adverse events occurred. Eight patients had at least 40% improvement in the PASI score, 6 at least 75% and 3 had more than 90% improvements, with a better trend in response in female patients. Three had no sign of disease at the physician global assessment. After administration, frequencies of regulatory T cells and CD4+ memory T cells were significantly increased, especially in responder patients, whereas the number of T helper 17, CD4+ naive T cells and serum level of interleukin 17, known to play a major role in psoriasis pathogenesis, were significantly decreased. These observations were in line with the hypothesis that MSCs would promote a more “immune tolerant” T cell phenotypes.
Conclusion: The authors concluded that allogeneic cord blood MSCs is safe and partially effective in psoriasis patients, and level of Tregs may be used as a biomarker to predict the clinical response.
Topical and Intravenous Administration of Human Umbilical Cord Mesenchymal Stem Cells in Patients with Diabetic Foot Ulcer and Peripheral Arterial Sisease: a Phase I Pilot Study with a 3-Year Follow-Up
Zhang C et al, Stem Cell Research and Therapy, 2022
The First Affiliated Hospital of Xi’an Jiaotong University, Shaanxi, China
The authors evaluated the safety and efficacy of cord blood-derived mesenchymal stem cells (MSCs) in patients with diabetic foot ulcer.
Methods: In this Phase I pilot study, 14 patients with peripheral artery disease and refractory diabetic foot ulcer were enrolled. Each patient received a single local injection and two systemic administrations of cord blood-derived MSCs. Adverse events were documented for safety assessments. The therapeutic efficacy was assessed by ulcer healing status and recurrence rate.
Results: No major adverse events were observed. Two patients developed transitory fever early after administration. Ulcers status was evaluated 1.5 month after treatment. The lesion severity decreased significantly in all patients, and complete closure was observed in 14 of the 15 lesions. Similarly, the symptoms of chronic limb ischemia were alleviated, despite that no improvement in obstruction was seen in computed tomography angiography imaging. Rehospitalization due to ulcer recurrence occurred in 1 patient within one year, and 5 within three years.
Conclusions: Authors concluded that MSC infusion was well tolerated and shortened ulcer healing time.
The Impact of GVHD on Outcomes after Adult Single Cord Blood Transplantation in European and Japanese Populations
Kanda J, et al, Bone Marrow Transplant, 2021
Kyoto University, Kyoto, Japan
The impact of GVHD and graft-versus-leukemia effect in unrelated cord blood transplantation (UCBT) is controversial.
Methods: In the Eurocord/ALWP EBMT and JSTCT/JDCHCT collaborative study, the impact of GVHD on UCBT outcomes in Japanese and European registries were evaluated. A total of 3,690 adult patients with acute leukemia who received their first single UCBT were included.
Findings: A multivariate analysis of overall survival (OS) revealed a positive impact of grade II acute GVHD compared with grade 0-I GVHD, in the Japanese cohort, and an adverse impact in the European cohort. A negative impact of grade III-IV acute GVHD on OS was observed regardless of registries. In the analysis of relapse, a positive impact of grade II acute GVHD compared with grade 0-I GVHD was observed only in the Japanese cohort, regardless of disease risk. The positive impact of limited chronic GVHD on OS was observed only in the Japanese cohort.
Conclusions: A positive impact of mild GVHD after a single UCBT was observed only in the Japanese cohort. This could explain the ethnic difference in UCBT outcomes and might contribute to the preference usage of UCBT in Japan.
Comparison of Haploidentical and Umbilical Cord Blood Transplantation after Myeloablative Conditioning
Wagner JE, et al, Blood Advances 2021
University of Minnesota, Minneapolis, Minnesota, USA
Most reports comparing haploidentical hematopoietic stem cell transplantation (haplo-HSCT) with posttransplant cyclophosphamide (PTCy) and other donor sources have focused on outcomes in older adults treated with reduced intensity conditioning.
Methods: The current study evaluated outcomes in patients with hematological malignancy treated with myeloablative conditioning prior to haplo- (n = 375) or umbilical cord blood (UCB; n = 333) HSCT.
Findings: All haplo recipients received a 4 of 8 HLA-matched graft, whereas recipients of UCB were matched at 6-8/8 (n = 145) or ≤5/8 (n = 188) HLA antigens. UCB recipients were more likely to have acute lymphoblastic leukemia and transplanted in second complete remission (CR), whereas haplo-HSCT recipients were more likely to have acute myeloid leukemia in the first CR. Survival at 3 years was similar for the donor sources (66% haplo- and 61% after ≤5/8 and 58% after 6-8/8 UCB). Notably, relapse at 3 years was lower in recipients of ≤5/8 UCB (21%, P = .03) compared with haplo- (36%) and 6-8/8 UCB (30%). However, non-relapse mortality was higher in ≤5/8 UCB (21%) compared with other groups (P < .0001).
Conclusions: Haplo-HSCT with PTCy after myeloablative conditioning provides an overall survival outcome comparable to that after UCB regardless HLA match group.
Engraftment of Double Cord Blood Transplantation after Nonmyeloablative Conditioning with Escalated Total Body Irradiation Dosing to Facilitate Engraftment in Immunocompetent Patients
Brunstein CG, et al. Transplantation and Cellular Therapy, 2021
University of Minnesota, Minneapolis, Minnesota, USA
It is unknown whether a higher dose of total body irradiation (TBI) could improve engraftment rate or other transplant outcomes for less heavily treated patients.
Methods: This was a secondary analysis of double unrelated cord blood (dUCB) recipients in BMT CTN 1101, 161 who received total body irradiation (TBI) 200 cGy and 18 who received TBI 300 cGy. In BMT CTN 1101, dUCB recipients who had not received cytotoxic chemotherapy within 3 months of enrollment or a previous autologous HCT within 24 months received TBI 300 cGy instead of 200 cGy
Findings: The probability of neutrophil engraftment was 100% for patients who received TBI 300 cGy versus 91% for those who received TBI 200 cGy (P = .64). There was no significant difference in the 1-year incidences of non-relapse mortality (NRM) and relapse or in 1-year survival. Patients who received TBI 300 cGy and 200 cGy had similar engraftment and early mortality.
Conclusions: Inclusion of a modified regimen for dUCB transplantation had no demonstrable influence on this large randomized trial.
Omidubicel vs Standard Myeloablative Umbilical Cord Blood Transplantation: Results of a Phase 3 Randomized Study
Horwitz ME, et al. Blood, 2021
Duke University Medical Center, Durham, North Carolina, USA
Omidubicel is an ex vivo expanded hematopoietic progenitor cell and nonexpanded myeloid and lymphoid cell product derived from a single umbilical cord blood unit.
Methods: The present study reports the result of a phase 3 trial to evaluate the efficacy of omidubicel compared with standard umbilical cord blood transplantation (UCBT). Between January 2017 and January 2020, 125 patients age 13 to 65 years with hematologic malignancies were randomly assigned to omidubicel vs standard UCBT. The primary end point was time to neutrophil engraftment.
Findings: Median time to neutrophil engraftment was 12 days for the omidubicel arm and 22 days for the control arm (P < .001). The cumulative incidence of neutrophil engraftment was 96% for patients receiving omidubicel and 89% for patients receiving control transplants. The omidubicel arm had faster platelet recovery, had a lower incidence of first grade 2 to 3 bacterial or invasive fungal infection, and spent more time out of hospital during the first 100 days after transplant than controls.
Conclusions: Transplantation with omidubicel results in faster hematopoietic recovery and reduces early transplant-related complications compared with standard UCBT. The results suggest that omidubicel may be considered as a new standard of care for adult patients eligible for UCBT.
Single Cord Blood Transplantation Versus Unmanipulated Haploidentical Transplantation for Adults with Acute Myeloid Leukemia in Complete Remission
Konuma T, et al. Transplantation and Cellular Therapy, 2021
The University of Tokyo, Tokyo, Japan
Comparative data for cord blood transplantation (CBT) and haploidentical related donor hematopoietic cell transplantation (haplo-HCT) are limited for adult patients with acute myeloid leukemia (AML) in complete remission (CR).
Methods: The allogeneic HCT in 1313 adult patients with intermediate- or poor-risk AML in CR who received either SCBT (n = 1102) or unmanipulated haplo-HCT (n = 211) between 2007 and 2018 in Japan were retrospectively analyzed.
Findings: Among the whole cohort, the cumulative incidences of neutrophil and platelet recovery were significantly lower in SCBT recipients compared with those in haplo-HCT recipients. SCBT was significantly associated with a higher incidence of grade II to IV acute GVHD and lower incidence of extensive chronic GVHD compared to haplo-HCT. Haplo-HCT recipients developed a higher incidence of CMV antigenemia compared to SCBT recipients (P = .004). In the multivariate analysis, there were no significant differences for grades III or IV acute GVHD, relapse incidence, non-relapse mortality, OS, LFS, GRFS, or CRFS between the two donor types.
Conclusions: SCBT and unmanipulated haplo-HCT had similar survival outcomes for adult patients with AML in CR despite the lower hematopoietic recovery and higher grade II to IV acute GVHD in SCBT recipients and the higher CMV antigenemia in haplo-HCT recipients.
A Multicenter Phase II Study of Intrabone Single-Unit Cord Blood Transplantation without Antithymocyte Globulin
Nishida T et al. Annals of Hematology, 2021
Nagoya University Graduate School of Medicine, Nagoya, Japan
Delayed engraftment is still an unmet issue in umbilical cord blood (UCB) transplants.
Methods: In this phase 2 study, authors investigated the use of intra-bone infusion of a single UCB unit without anti-thymocyte globulin to overcome this issue.
Results: 62 patients were analysed; median UCB cellularity was 2.57 × 107/kg of total nucleated cells. Median time to neutrophil ≥ 500/ µL was 21 days; at day60, cumulative incidence of neutrophil engraftment was 80.6% (95% CI 68.2%-88.6%). Median time to platelet ≥ 20.000/ µL was 38 days, at day100 cumulative incidence was 75.8% (62.6-84.9%). Day100 cumulative incidence of grade III-IV acute GvHD was 6.5%, without steroid-refractory cases, whereas 1-year cumulative incidence of chronic GVHD was 9.9%.
Conclusions: The authors concluded that intra-bone infusion of single UCB unit was safe and feasible.
Allogeneic Stem Cell Transplantation with Omidubicel in Sickle Cell Disease
Parikh S et al. Blood Advances, 2021
Duke University School of Medicine, Durham, North Carolina, USA
Historically, patients with sickle cell disease transplanted with umbilical cord blood (UCB) had unacceptable rate of graft failure.
Method: In this phase 1/2 study the authors aimed to evaluate engraftment rate in 13 patients transplanted with omidubicel in combination with an unmanipulated UCB and in 3 patients transplanted with a single omidubicel graft.
Results: Median neutrophil engraftment occurred at 7 days. Long-term engraftment was derived from the unmanipulated graft in 10/13 of the double graft recipients. Two of the three single omidubicel recipients also had sustained engraftment.
Conclusions: Authors concluded that omidubicel transplant determined rapid engraftment in this population, who is known to have intrinsic resistance to engraftment, and that this approach deserves further investigations also in other non-malignant disorders, including bone marrow failures.
Umbilical Cord Mesenchymal Stem Cells for COVID-19 Acute Respiratory Distress Syndrome: A Double-Blind, Phase 1/2a Randomized Controlled Trial
Lanzoni G et al. Stem Cells Translational Medicine, 2021
University of Miami Miller School of Medicine, Miami, Florida, USA
Mesenchymal stem cells’ anti-inflammatory properties might be effective in acute respiratory distress syndrome in patients with COVID-19
Methods: in this double blind, randomized phase 1-2 study, the authors evaluated safety and feasibility of intravenous infusion of 100 ± 20 × 106 UCB-derived MSCs in COVID-19 patients stratified by acute respiratory distress syndrome severity.
Results: MSCs infusion was well tolerated, it determined a rapid decrease in inflammatory cytokines, and was associated with improved survival (91% vs 42%, p 0.015).
Conclusion: UC-MSC infusions are safe and could be beneficial in treating subjects with COVID-19-related acute respiratory distress syndrome.
Umbilical Cord Mesenchymal Stromal Cells as Critical COVID-19 Adjuvant Therapy: A Randomized Controlled Trial
Dilogo IH, Stem Cells Translational Medicine, 2021
Medicine Universitas Indonesia, Jakarta, Indonesia
Umbilical cord blood-derived mesenchymal stromal cells are known to favor a more anti-inflammatory microenvironment.
Methods: The authors conducted a double-blind, multicentre study, where 40 patients were randomized to receive standard therapy with or without an endovenous administration (1 × 106/kg) of UCB-derived MSCs; primary endpoint was overall survival and length of assisted ventilation need.
Results: Infusion was well tolerated. Patients who received MSC had a survival rate 2.5 times higher compared to the control group, although no difference were seen in need of assisted ventilation.
Conclusion: The authors concluded that UCB-derived MSCs might be effective as an anti-inflammatory agent in patients with severe COVID-19.
Clinical and Imaging Outcomes after Intrathecal Injection of Umbilical Cord Tissue Mesenchymal Stem Cells in Cerebral Palsy: a Randomized Double-Blind Sham-Controlled Clinical Trial
Amanat M et al. Stem Cell Research & Therapy, 2021
University of Medical Sciences, Tehran, Iran
In this study, authors assessed safety and efficacy of intrathecal injection of umbilical cord blood-derived MSC in patients with spastic cerebral palsy.
Methods: 72 pediatric patients with cerebral palsy were randomized to receive either a single 2×107 MSC dose or placebo. The study primary endpoint was changes in clinical scores that measure gross motor function.
Results: Compared to controls, patients in the study group had significant measurable improvements in gross motor function, as well as in quality of life.
Conclusions: Authors concluded that intrathecal injection of UCB-derived MSC was safe and effective in this population and deserves further investigation.
Repeated Subarachnoid Administrations of Allogeneic Human Umbilical Cord Mesenchymal Stem Cells for Spinal Cord Injury: a Phase 1/2 Pilot Study
Yang Y et al. Cytotherapy, 2021
Guangdong Provincial Center for Engineering and Technology Research of Minimally Invasive Spine Surgery, Guangzhou, China
Umbilical cord blood-derived mesenchymal stem cells are a potential treatment for severe spinal cord injury.
Methods: In this prospective trial, 102 patients with severe spinal cord injury were enrolled to receive up to four, monthly subarachnoid infusion of UCB-derived MSCs (1 × 106 cells/kg). Clinical and radiological scores were used to evaluate efficacy.
Results: No severe adverse events were observed. Authors reported significant improvement in sensory motor skills as well as autonomic function, regardless the severity and level of spinal injury.
Conclusion: Authors concluded that intrathecal administration of UCB-derived MSCs was well tolerated and significantly improved neurological dysfunction.
Cord Blood Highlights from the 2025 Tandem Transplant Meeting in Hawaii
Warren Fingrut, MD
The 2025 Tandem Meetings of the American Society for Transplantation and Cellular Therapy (ASTCT) and Center for International Blood and Marrow Transplant Research (CIBMTR) in Honolulu, Hawaii (February 12-15, 2025) included a spotlight session outlining new frontiers cord-blood therapies.
First, Dr. Colleen Delaney from Fred Hutchinson Cancer Research Center, USA spoke about leveraging cord blood stem cell expansion for the generation of multiple off-the-shelf immunotherapy products. Dr. Delaney reviewed the cell and gene therapy landscape, highlighting the major role of cord-blood derived therapies, and discussed the promise of these therapies as well as challenges limiting their development and use. Her talk outlined how cord blood is an attractive source of starting material for cell therapies, and shared data demonstrating the safety of infusing cord blood derived-products that are from pooled donors and which are not human leukocyte antigen (HLA)-matched to the recipient.
Next, Dr. Partow Kebriaei from The University of Texas MD Anderson Cancer Center reviewed cord tissue derived mesenchymal stromal cells (MSCs) for graft-versus-host disease (GVHD) and regenerative medicine. She highlighted that, currently, there are over 1400 active clinical trials registered on ClinicalTrials.gov which are studying clinical applications of MSCs. She shared data outlining that cord blood derived MSCs have unique properties compared with MSCs derived from bone marrow, and showed how cord blood derived MSCs are manufactured at the MD Anderson GMP facility. Dr. Kebriaei then discussed three active trials of cord blood-derived MSCs at MD Anderson, studying their therapeutic roles in steroid-refractory acute GVHD, acute respiratory distress syndrome (ARDS), and cardiac toxicity resulting from anthracycline chemotherapy.
Dr. Takanori Teshima from Hokkaido University Faculty of Medicine, Sapporo, Japan spoke about optimized cord blood transplantation practices in Japan. He explained that cord blood remains a major source for allogeneic stem cell transplantation in Japan, with 38% of transplant recipients there receiving cord blood grafts. He reviewed Japanese data that has guided optimization of cord blood transplantation conditioning chemotherapy, GVHD prophylaxis, and unit selection.
The next presentation was delivered by Dr. Guy Sauvageau from the Institute for Research in Immunology and Cancer (IRIC) at University of Montreal, Montreal, Canada. Dr. Sauvageau spoke about the epigenetic rejuvenation of stem cells for better transplants. He outlined the properties of young, balanced hematopoietic stem cells which are present in cord blood grafts, and discussed approaches under investigation to harness these stem cells. He reviewed the UM171 treatment, including results from phase II clinical trials studying this therapy in patients with high-risk acute myeloid leukemia (AML).
Finally, Dr. Rafet Basar from the laboratory of Dr. Katy Rezvani at The University of Texas MD Anderson Center Center spoke about cord blood derived natural killer (CB-NK) cells for cancer therapy. Dr. Basar shared data highlighting the use of chimeric antigen receptor (CAR) transduced CB-NK cells in lymphoid malignancies. He outlined ongoing clinical trials studying the safety and efficacy of CAR-NK cells targeting CD70 and CD5 in patients with a range of hematologic malignancies which express these antigens.
SELECTED ABSTRACTS: 2024 Cord Blood ASH meeting
Comparison of Myeloablative Conditioning with a Total Body Irradiation-Based Regimen Versus a Chemotherapy-Based Regimen in Adult Patients Undergoing Umbilical Cord Blood Stem Cell Transplant for Ph-Negative Acute Lymphoblastic Leukemia
Jason S. Gilbert, Andrew Kent, Jonathan Gutman, Maria L Amaya, Christine M. McMahon, Daniel A Pollyea, Marc Schwartz , Department of Medicine, University of Colorado School of Medicine, Aurora, CO
Objective: To compare outcomes of adult patients with ALL who underwent cord blood transplant with a high dose TBI-based regimen versus a myeloablative chemotherapy-based regimen.
Methods: Single center, retrospective study of adult patients with patient with acute lymphoblastic leukemia who received a stem cell transplant using a high intensity conditioning regimen. Nineteen of the 36 patients received a chemotherapy-only conditioning regimen and 17 received total body irradiation as part of the conditioning regimen.
Findings: Outcome measures such as survival without relapse, or survival in general (overall survival) were superior among those who received total body irradiation as part of their conditioning regimen.
Conclusions: This is a small study done at a single institution, so firm conclusions cannot be made. However, the current standard of care in the United States is to use total body irradiation for patients with acute lymphoblastic leukemia getting an umbilical cord blood transplantation. This study suggests that this standard of care should continue.
The influence of HLA Matching of the Winning and Losing Units in Relapse of Patients Receiving Double Cord Blood Transplantation for Acute Leukemia or Myelodysplastic Syndrome
Robert F. Wynn, Fernanda Volt, Srividhya Senthil, Graziana Scigliuolo, Chantal Kenzey, Barbara Cappelli, Annalisa Ruggeri, Ryad Tamouza, Vanderson Rocha, Li Yuan Chan, Ka Lok Chan, Chloe Anthias, Regis Peffault De Latour, Ben Carpenter, Adrian Bloor, Marc Bierings, Nicolaas Schaap, Persis Jal Amrolia, Jan J. Cornelissen, Kay Poulton, Éliane Gluckman
On behalf of the European Blood and Marrow Transplant Registry
Objective: When a patient receives a double unit cord blood transplant, one unit typically “wins” and becomes the predominant source of blood cell production. This study examines whether the degree of matching between the winning unit and the patient impacts the chance for post-transplant recurrence of disease
Methods: Large, registry-based study of children and adult patients receiving double umbilical cord blood transplantation in Europe.
Findings: The winning unit typically “rejects” the losing unit through an HLA-restricted response to mismatched HLA-A or HLA-B alleles. As part of this response, the losing unit primes the winning unit anti-tumor response resulting in a reduced risk for disease recurrence.
Conclusions: This study suggests that the selection of umbilical cord blood units used in a double cord blood transplant may influence the likelihood of a successful outcome.
Efficacy and Safety of TAK-007, Cord Blood-Derived CD19 CAR-NK Cells, in Adult Patients with Relapsed/Refractory (R/R) B-Cell Non-Hodgkin Lymphoma (NHL)
Justin M. Darrah, MD 1, Indumathy Varadarajan 2, Amitkumar Mehta, MD 3, Jennifer N. Saultz, DO 4, Matthew McKinney, MD 5,5, Monalisa Ghosh, MD 6, Usama Gergis, MDMBA 7, Girish Bende 8, Siddha Kasar, PhD 8, Bradley Hupf 8, Sharon Chen 8, Leopold Sellner 8, Reem Karmali, MD 9
- Division of Hematology and Cellular Therapy, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA
- University of Virginia, Charlottesville, VA
- University of Alabama at Birmingham, Birmingham, AL
- Knight Cancer Institute, Oregon Health & Science University, Portland, OR
- Duke Cancer Institute, Durham, NC
- University of Michigan, Ann Arbor, MI
- Thomas Jefferson University, Philadelphia, PA
- Takeda Development Center Americas, Inc. (TDCA), Lexington, MA 9 Northwestern Memorial Hospital, Chicago, IL
Objective: Chimeric Antigen Receptor-T cells (CAR-T) have revolutionized the treatment of Non-Hodgkins lymphoma. Procurement of this patient-specific cell therapy is complex, prolonged and extremely expensive. An off-the-shelf alternative that is equally effective is needed. This allogeneic CAR-NK cell product has the potential to address this clinical need.
Methods: An open-label, multi-center phase 2 trial was conducted to evaluate the safety and preliminary efficacy the CAR-NK product TAK-007 in adult patients with relapsed/refractory large B cell lymphoma or indolent non-hodgkin lymphoma.
Findings: The results of 27 subjects are reported. Cytokine release syndrome, the most common immune-related complication of CAR-T treatment, was mild. There was no neurotoxicity. 50% of subjects receiving the larger cell dose had a response to therapy.
Conclusions: Off the shelf CD19 directed CAR-NK cells (TAK-007) is a promising allogeneic cell therapy product. Further studies are needed.
SELECTED ABSTRACTS: 2023 Cord Blood Connect International Congress
Umbilical Cord Blood Treatment to Improve Gross Motor Function in Individuals with Cerebral Palsy: Results from an Individual Participant Data Meta-Analysis
Megan Finch-Edmondson, et al
Cerebral Palsy Alliance Research Institute, The University of Sydney, Australia
Umbilical Cord Blood Transplantation (UCBT) is an emerging treatment for cerebral palsy.
Methods: A meta analysis was conducted looking at 9 published and 2 unpublished studies, including 7 randomized clinical trials.
Findings: 285 patients and 171 controls participated in these studies. Most studies used allogeneic (donor) UCB and gave the UCB intravenously. At 6 months after UCBT, the Gross Motor Function Measure improved 0.93 points greater than the controls. Higher cell doses were associated with improved increases in Gross Motor Function.
Conclusions: UCBT improves gross motor function in patients with cerebral palsy. Higher cell doses appear to be beneficial.
Long-Term Stability of Cord Blood Units after > 29 years of cryopreservation: follow-up data from the Jose Carreras Cord Blood Bank
Stefanie Liedtke, et al
Heinrich-Heine-University Dusseldorf, Jose Carreras Cord Blood Bank
The Jose Carreras Cord Blood Bank has over 21,000 umbilical cord blood (UCB) units and has released 1476 UCB units for Umbilical Cord Blood Transplant (UCBT).
The study analyzes the stability of units under long term storage.
Methods: A retrospective analysis of UCB units processed by either manual methods (1998-2005) or by Sepax method (after 2005) was performed.
Findings: Total nucleated cell (TNC) recovery was 97% and TNC viability was 88% after 25 years of cryopreservation.
Conclusions: Long term storage of UCB units shows excellent recovery of total nucleated cells and viability.
SELECTED ABSTRACTS: 2023 EBMT Annual Meeting
Robert Wynn – optimizing AML outcomes using CB in UK experience.
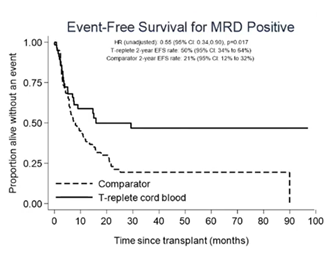
Dr Wynn compared the outcomes of 112 children with acute myeloid leukemia (AML) undergoing T cell replete CB transplant versus 255 AML children who received transplant from other stem cell source, including matched unrelated donors (n=136), sibling donor (n=63), mismatched unrelated donor (n=36), haploidentical (n=11) and T cell deplete CB (n=9). All patients were transplanted in the same time frame, between 2014 and 2021. When patients were stratified by the minimal residual disease (MRD) status at the time of the transplant, no significant difference was seen between the two groups in terms of event free survival (EFS) among patients transplanted with a negative MRD. However, patients transplanted with a detectable MRD had a higher probability of long-term remission if they had received a T cell replete CB transplant (Figure): 2-year EFS 50% vs 21%, p=0.017 and relapse rate 36.2% vs 66.2%, p=0.007, confirming the data reported by other groups (Milano et al, 2016). Overall survival did not differ significantly between CB and non-CB cohort due to higher transplant-related mortality in the CB cohort, again remarking the need to work toward improving management of transplant-related complication. Dr Wynn also presented a prospective trial that will open in late 2023 that will randomize paediatric patients with AML in CR1 or CR2 to receiving expanded CB versus non-CB graft sources (expansion will use UM171 platform, as previously described by Cohen, Lancet Hametol 2020).
Dr Wynn reported preliminary data on trial NCT05425043, which adopt third party granulocyte transfusion early after CB transplant to accelerate immune recovery in patients with relapsed/refractory diseases who failed a previous BMT. Seven doses of granulocytes infusions are planned according to trial design, starting from day +1 after CBT. Ten patients have been treated so far; granulocyte infusions were overall well tolerated, with some degree of cytokine release syndrome and, importantly, transient robust donor derived CD8 T cell expansion, which could potentially implement leukemic cell eradication, as suggested by pre clinic data. Five patients from the trial are still in complete remission, four died of disease relapse and one due to transplant-related complication.
Vinod K Prasad – Newborn screening for Hurler syndrome leads to early treatment and excellent outcomes with unrelated umbilical cord blood transplant.
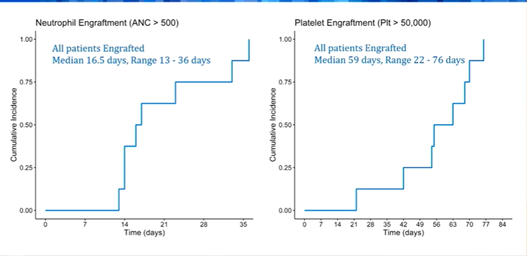
Dr. Prasad presented the data of CB transplants in children diagnosed with Hurler syndrome, a rare autosomal recessive, lysosomal storage disorder that is associate with early, progressive developmental delay, skeletal abnormalities, cognitive impairment, cardiovascular and respiratory problems. Early transplant, possibly at 4-5 months of age, is recommended to maximize the survival outcomes. A total of 10 patients were treated with CB transplants; all but three were below six months of age at the time of the transplant. Enzyme level prior to CBT was undetectable in all but one patient. All patients received a busulfan based, ATG containing conditioning regimen. Patients were followed for a median of 2.4 years (range 0.2 – 6.1). Engraftment rate of favourable (Figure): neutrophils (>500/mmc) and platelet (>50,000/mmc) engraftment occurred in all patients after a median of 16 and 56 days, respectively. All patients achieved complete donor chimerism with normal enzyme level at last evaluation. None experienced graft failure. Complications included veno-occlusive disease (n=2), haemolytic anemia (n=3, one of which was fatal), and microangiopathy (n=1). None developed severe acute or chronic GvHD. The authors concluded that children with Hurler syndrome can be successfully treated with CBT with favourable outcomes.
Elizabeth Hicks - Outcomes of UCB transplant in children with chronic granulomatous disease
In this poster presentation, Dr Hicks reported the outcomes of 19 children with CGD treated between 2005 and 2022. Median age at transplant was 2.4 years. All but one had prior history of bacterial and fungal infection, in most cases with recurrent episodes, including pneumonia, cellulitis, and enteritis. In three cases, CB was from a related donor. HLA matching include 4/6 (n=5), 5/6 (n=12) and 6/6 (n=2). All received busulfan based, myeloablative conditioning regimen, with ATG. After a median follow up of 6 years, 16 patients are still alive and disease free; two died due to transplant-related cause, and one for Duchenne syndrome, undiagnosed at the time of the transplant. Three patients had graft failure which required a subsequent transplant. None developed CGD-related infection after transplant. Two patients had high grade acute GVHD, whereas two experienced extensive chronic GVHD. The authors concluded supporting the use of CB in paediatric patients with CGD lacking HLA identical donor.
Vanderson Rocha – is there a role of CB in pediatric setting in the future?
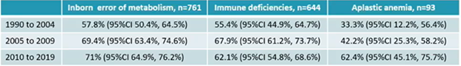
Dr Rocha reported registry data from Eurocord and Duke University, which collected data from a large cohort of 1995 children who underwent CB transplant between 1993 and 2019 for a non-malignant disorder. The aim of this retrospective study was to evaluate how did CB transplant outcomes change over the course of the time.
Decades 1990-2004, 2005-2009 and 2010-2019 were analysed separately, as well as primary disease (inborn errors, primary immunodeficiency and aplastic anaemia). In all three disease-category, survival outcomes significantly improved overtime (Table), mostly due to better knowledge of CB selection and post-transplant management. Regardless timeframe, a trend toward a better outcome was seen in ATG-free and myeloablative conditioning setting, as well as with younger patient age, better patient-CB HLA matching and negative patient CMV serostatus.
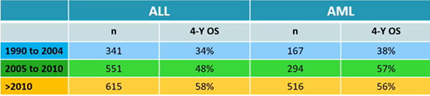
Similar analysis was performed for 3205 paediatric patients with malignant disorders, divided per myeloid or lymphoid leukemia. Also in this setting, patients transplanted after 2010 had a significant survival improvement. Use of total body irradiation and myeloablative conditioning also had a favourable impact.
The author concluded by remarking the favourable outcomes in terms of disease relapse and long-term complications (e.g. chronic GVHD) in selected patients category undergoing CB transplant.
SELECTED ABSTRACTS: 2022 Cord Blood Connect International Congress
Safety of DUOC-01, Intrathecal Cord Blood-Derived Cellular Therapy, as an Adjunct to Allogeneic Cord Blood Transplantation in Children with Inherited Metabolic Diseases
Sun J et al. Duke University, Durham, North Carolina, USA
Unrelated umbilical cord blood transplantation (UCBT) improves function and extends life in children with inherited metabolic disorders. Neurologic disease can progress after UCBT, prior to donor engraftment in the brain. DUOC-01 is a monocyte-derived cord blood cell product, designed to accelerate donor cell delivery to the brain.
Methods: DUOC-01 was manufactured from cord blood units. Cells were given intrathecally. Forty children, ages 0-15, received umbilical cord blood transplant. Thirty patients received DUOC-01 cells intrathecally at a median of 31 days post-transplant.
Results: Two patients developed transient fever and hypotension post infusion. No other adverse events related to DUOC-1 were reported.
Conclusion: DUOC-1 is a cord blood-derived product intended to accelerate donor cell delivery to the brain of children with inherited metabolic diseases undergoing cord blood transplant. It is well tolerated. Further studies are underway to determine efficacy.
Development of CAR-NK Cell Therapy for Hematologic Malignancies
Stoltzman C et al. Deverra Therapeutics and Fred Hutchinson Cancer Center, Seattle, Washington, USA
Chimeric antigen receptor (CAR) expression by natural killer (NK) cells may improve the potency and specificity for the NK cells anti-tumor efficacy. NK CAR cells may be a safer and more cost-effective way to deliver CAR T cell therapy.
Methods: CD 56+ NK cells are generated from cord blood-derived NK cells. The cells are transduced with a viral vector that targets a protein specific for acute lymphoid leukemia (ALL). A mouse model was used to test the cell product.
Results: The CAR-NK cell product was safely given in up to 8 repeat doses to mice with ALL. The mice had inhibited tumor growth and improved survival compared with the control mice, who received a control NK product.
Conclusions: Cord blood-derived CAR-NK cells can be manufactured and have specific potency vs ALL in a mouse model. Further preclinical studies are planned in other blood cancers.
A Randomized, Placebo-Controlled, Phase II Trial of Intravenous Allogeneic Umbilical Cord Blood Infusion for Adults with Ischemic Stroke
Laskowitz D et al. Duke University, Durham, North Carolina, USA
Stroke is the 5th leading cause of death in the United States, and current treatments are not effective in all patients. The purpose of this study was to determine if infusion of a non-HLA matched unrelated umbilical cord blood unit (UCB) unit can improve outcomes.
Methods: A Phase II, multicenter, randomized, placebo-controlled study was performed in several U.S. centers. Cord blood units were infused 3-10 days post stroke. The primary endpoint was a change in the Modified Rankin Scale, a scale of functional activity.
Results: 79 patients were enrolled at 6 centers. The trial was closed early due to slow accrual attributed to COVID. There were 17 mild infusion reactions. There was no difference in the Modified Rankin Scale between patients who received and who did not receive the cord blood units.
Discussion: UCB can be given safely after stroke. There was no difference in the primary endpoint in this small study. A larger study with more functional outcomes could be considered.
Should Cord Blood Unit Distribution Patterns Impact Collection Strategies
Frenet E et al. New York Blood Center, New York, New York, USA
The National Cord Blood Program has an inventory of more than 60,000 cord blood units for clinical transplant use. This study characterizes the current inventory and the distributed cord blood units. It recommends the collection strategy moving forward.
Methods: 1,046 shipments of unrelated cord blood (UCB) from 2015 to 2022 were studied. Race/ethnicity, HLA match and cell dose information was reviewed.
Results: Non-Hispanic White (White) units represented 46% of the inventory and 38% of the distributed units. Match levels were higher among White units (55% 5/8 or greater) than for units collected from other race/ethnicities (40% 5/8 or greater). The median total nucleated cell dose was 167 x 10 (7) for transplanted cord blood units and 102 x 10 (7) for stored cord blood units.
Conclusions: White cord blood units are distributed most frequently, and had a higher allele match to the recipient. The goal for new collections should be to obtain UCB units with higher total nucleated cell and CD 34 counts.
SELECTED ABSTRACTS: 2022 Transplantation and Cellular Therapy Tandem Meetings
Hematopoietic Stem Cell Transplantation (HSCT) with Omidubicel Is Associated with Enhanced Circulatory Plasmacytoid Dendritic Cells (pDC), NK Cells and CD4+ T Cells with Lower Rates of Severe Infections Compared to Standard Umbilical Cord Blood Transplantation
Szabolcs P et al. University of Pittsburg, Pittsburg, Pennsylvania, USA
Methods: This was a sub-study of a recently reported randomized Phase III study comparing myeloablative transplantation with omidubicel versus standard umbilical cord blood transplantation. Samples and data were obtained from 17 subjects transplanted with omidubicel and 20 patients transplanted with standard umbilical cord blood grafts.
Results: At D+7 and D+14 post-transplant, CD3+, CD4+ T cells, and Tregs were significantly higher in omidubicel patients than in controls. B cells and NK cells, as well as monocytes, myeloid and plasmacytoid DC, were also higher in omidubicel patients.
Conclusion: Omidubicel recipients experience more rapid recovery of multiple lymphoid subsets, which may contribute to a reduction in viral infections recipients of omidubicel, compared to standard umbilical cord blood transplantation.
A Phase I Study of Allogeneic Umbilical Cord Tissue Derived Mesenchymal Stromal Cells for Term and Near-Term Infants with Hypoxic-Ischemic Encephalopathy (HIE)
Cotton M et al. Duke University, Durham, North Carolina, USA
Methods: This is a Phase I study of allogeneic umbilical cord tissue-derived mesenchymal stromal cells given to infants with hypoxic-ischemic encephalopathy.
Results: 6 infants were enrolled, 4 with moderate and 2 with severe hypoxic-ischemic encephalopathy. No infusion reactions were observed. While one product was culture positive for coag-negative staphylococci, no subjects contracted an infection. 5 infants were evaluable for neurocognitive studies at 12-16 months of age. For the 5 evaluable subjects, cognitive, language and motor composite scores ranged from 90 – 100, 83 – 109, and 82 – 115, respectively.
Conclusion: Allogeneic human cord tissue-derived mesenchymal stromal cells can be safely administered to infants with hypoxic-ischemic encephalopathy.
Bone Marrow and Umbilical Cord Blood Are Equivalent Stem Cell Sources for Hurler Syndrome
Lund P et al. University of Minnesota, Minneapolis, Minnesota, USA
Methods: The investigators compared leukocyte lysate and plasma iduronidase activity in patients with Hurler Syndrome who had received umbilical cord blood (n=20) and bone marrow transplantation (n=9).
Results: There was no difference among the leukocyte or plasma IDUA levels between umbilical cord blood and bone marrow recipients (18.7 vs 16.4 nmol/hr/mg, p=0.58 and 4.5 vs 4.5 nmol/hr/mg, p=0.98, respectively)
Conclusion: It has been proposed that umbilical cord blood should be the preferred source of stem cells in patients with Hurler Syndrome undergoing allogenic stem cell transplantation. The results of this study suggest that bone marrow grafts could be equally effective in restoring iduronidase activity.
Extended Letermovir Prophylaxis Is Highly Effective Cytomegalovirus (CMV) Infection Prevention in Adult Cord Blood Transplantation (CBT) Recipients and Does Not Prevent Emergence of CMV-Specific Donor-Derived Immunity
Politikos I et al. Weill Cornell Medical College, New York, New York, USA
Methods: The investigators report the efficacy and toxicity of a 6-month course (instead of the standard 3-month course) of letermovir as prophylactic treatment against cytomegalovirus in recipients of umbilical cord blood transplantation.
Results: 28 consecutive umbilical cord blood transplant recipients were enrolled, and 21 disease-free patients were evaluable after a 6-month course of letermovir prophylaxis. None of these patients experienced a clinically significant CMV infection. Following discontinuation of letermovir, 5 of 21 patients experienced a clinically significant CMV infection.
Conclusion: A prolonged course of letermovir is safe and efficacious in preventing clinically significant CMV infections. However, late infections after discontinuation are common, highlighting the need for prolonged CMV monitoring.
|